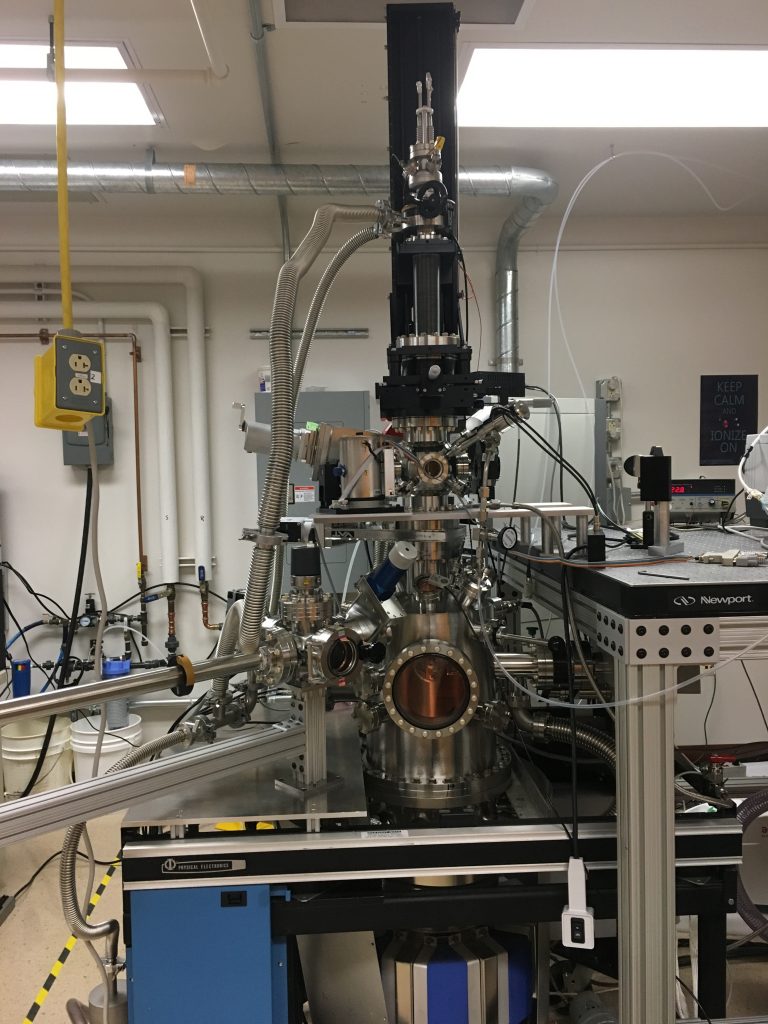
Perrine Group Surface Analysis Instrument
Surface science provides the foundation for understanding chemical mechanisms and physical transformations on surfaces. Multiple surface analytical techniques are needed to uncover interfacial chemical reactions by addressing questions:
- how molecules adsorb and react with surfaces
- identification of surface intermediates
- what products evolve from the surface
- how does the surface restructure during a reaction
Surface science has evolved from measuring reactions under controlled conditions in ultra-high vacuum (UHV) towards ambient pressure conditions, while retaining surface sensitivity. Low pressures are needed to controllably introduce a reactant (in the gas phase) to a surface. This is fulfilled by having a low base pressure (< 1×10-9 Torr) in order to prevent undesired adsorption (from air) with the surface. Modern surface science has shifted that pressure regime towards reaction conditions (mTorr-Torr), while still retaining the surface sensitivity in the measurement process. Reactions can be measured between -160 C to 900 C.
The spectroscopies on our Surface Analysis instrument will measure how adsorbates and their intermediates transform on a surface. *located at 615 Minerals & Materials Building
The Perrine Research Group Surface Analysis Instrument includes:
 Polarized Modulated-Infrared Reflection Absorption Spectroscopy (PM-IRRAS and IRRAS) and ATR-FTIR spectroscopy
Polarized Modulated-Infrared Reflection Absorption Spectroscopy (PM-IRRAS and IRRAS) and ATR-FTIR spectroscopy
A surface sensitive FTIR technique that utilizes polarization and modulation of infrared light to measure the surface intermediate species at a high grazing angle. This technique measures surface vibrational species that help us measure chemical reactions in UHV up to ambient pressures and at the air/liquid/solid interface. The surface species are detected by measuring the infrared vibrational spectra as a function of adsorbate coverage or at different temperatures to determine adsorption kinetics or decomposition of products.
Auger Electron Spectroscopy (AES)
AES is an electron spectroscopy technique that allows measurement of chemical states and binding of elements on a surface before and after surface adsorption. The AES process involves ejecting a secondary electrons from core-levels using electron emission.
Low Energy Electron Diffraction (LEED)
LEED uses low energy electrons to measure the diffraction patterns from the top layers of a surface. This technique measures the changes in surface structure and reconstruction before and after chemical adsorption.
Temperature Programmed Desorption (TPD)
TPD utilizes a mass spectrometer to detect the partial pressures of the mass fragments, from molecular adsorbates that desorb from a surface. The desorption peak and the shape of the curve yield information on the desorption kinetics, surface sites and reaction mechanisms. This technique is highly complementary to IRRAS to connect desorption energies with surface adsorbed species.
Chemical Wet Lab
Laboratory space for ex situ sample preparation is available to support our surface chemical preparation methods. This space includes 2 hoods, bench top space, furnaces, flammable storage cabinet, and extra vacuum equipment. *located at 711 Chemical Sciences and Engineering Building
Shared Facilities we use at Michigan Technological University
 X-ray Photoelectron Spectroscopy (XPS)
X-ray Photoelectron Spectroscopy (XPS)
XPS is a quantitative surface science technique that measures surface elemental composition, binding energies and electronic structure of both the surface material and adsorbed molecules. The PHI 5800 XPS in ACMAL is equipped with a dual source anode (Al and Mg), a hemispherical analyzer, for XPS and AES analysis, including elemental mapping capabilities, an electron gun source for AES analysis, an ion sputter gun for depth profiling and stage tilting for angle-resolved XPS.
The XPS was generously donated by the Army Research Laboratories in 2016 with help from former Chair Cary Chabalowski, Department of Chemistry at Michigan Tech with coordinated efforts by Dr. Perrine and ACMAL Director Owen Mills.
*located at 6th floor Minerals & Materials Building ** We work with Dr. Timothy Leftwich (pictured) in the Department of Materials Science & Engineering on select projects using XPS.
Atomic Force Microscopy (AFM)
AFM measures the surface topography and roughness of our surfaces to connect the surface chemical changes from spectroscopy to microscopic changes. This technique enables imaging features on the nano and atomic scale in air and in solution. We use tapping (AC) mode for basic imaging in air, imaging in liquids, and conductive AFM.
*funded by the NSF MRI grant #CHE 1725818); Dr. Perrine was a co-PI, *located and supported in ACMAL, 6th floor M&M building
Atomic Layer Deposition (ALD)
ALD is a chemical vapor deposition technique that allows for chemical growth of materials one atomic layer at a time. A metal-organic precursor and an oxidative co-reactant are used to deposit conformal films on surfaces. We used this technique to grow tailored architectures for unique materials for the next-generation heterogeneous catalysts or as model systems for environmental studies. This instrument was refurbished by the Microfabrication Facility and added to their suite of instruments. *located at 436 M&M Building
**This instrument effort was in collaboration with Dr. Chito Kendrick, Prof. Paul Bergstrom and Prof. Joshua Pearce of the Department of Electrical and Computer Engineering, at Michigan Tech
Other Shared Facilities we use at Michigan Technological University
The Applied Chemical and Morphological Analysis Laboratory (ACMAL) is a University Shared Facility which is part of the Materials Characterization & Fabrication Facilities. ACMAL houses an extensive array of electron microanalytical and X-ray instruments. ACMAL is managed by the Department of Materials Science and Engineering. *located at 6th floor Minerals & Materials Building
- Scanning Electron Microscopy
- Atomic Force Microscopy
- Scanning Transmission Electron Microscopy
- X-ray Diffraction
The Microfabrication Facility (MFF) is Michigan Tech’s resource for micro- and nano-scaled research and development of solid state electronics, micro electro mechanical systems (MEMS), and microsystems materials and devices. The ALD instrument will be added to the suite of instruments in the MFF. *located at 4th floor Minerals & Materials Building
Other instruments are available within the Department of Chemistry at Michigan Tech: UV-VIS spectroscopy, GC-MS, LC-MS, NMR
Off campus instruments: Ambient Pressure-XPS
We collaborate with scientists at the Advanced Light Source at Lawrence Berkeley National Laboratory as general users to run special experiments to corroborate our experiments at Michigan Tech.