Targeting metabolically-compromizes cells via carbohydrate transporters
The necessity in targeted therapies stimulates the search for cellular components or pathways that differ between normal vs. tumor cells and can serve as therapeutic targets or platforms for drug delivery. One of the aspects that lies within the interests of our research groups is enhanced nutrient uptake and expression of transmembrane transporters observed in cancers. Particular attentions is given to fructose transport that appears to be a good marker for cells that undergo transformation in to the neoplastic stage, or for tumors that grow and metastasize. Enhanced fructose uptake is also present in obesity and type II diabetes, a nonalcoholic fatty liver disease, gout, sclerosis and Alzheimer’s, suggesting the link between impaired metabolism and disease development.
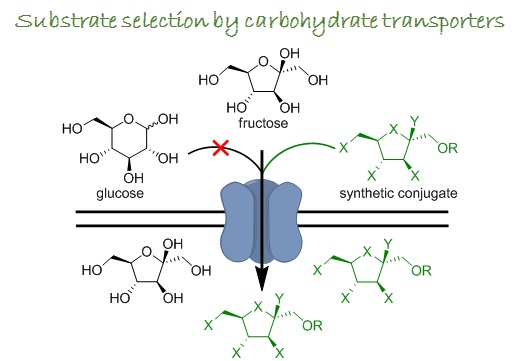 Despite the recognition of the role that fructose plays in human health, there remains an unfilled gap in understanding the relationship between fructose metabolism and disease onset/progression and whether blocking fructose uptake/metabolism can force metabolically compromised cells to become apoptotic. Evaluating these possibilities, as well as getting access to straightforward quantification of fructose uptake and metabolism as biomarkers of malignancy is currently limited due to the absence of probes to target fructose transport specifically. Our research group is working in the direction of understanding substrate selection by fructose transporters and obtaining fructose transport-specific probes for bioanalytical and biomedical applications.
Despite the recognition of the role that fructose plays in human health, there remains an unfilled gap in understanding the relationship between fructose metabolism and disease onset/progression and whether blocking fructose uptake/metabolism can force metabolically compromised cells to become apoptotic. Evaluating these possibilities, as well as getting access to straightforward quantification of fructose uptake and metabolism as biomarkers of malignancy is currently limited due to the absence of probes to target fructose transport specifically. Our research group is working in the direction of understanding substrate selection by fructose transporters and obtaining fructose transport-specific probes for bioanalytical and biomedical applications.
Design and synthesis of bioreductively-activated DNA repair inhibitors
Conventional chemotherapy drugs act by damaging DNA in tumor cells and triggering cell death. One important factor that defines the outcome of treatment with DNA damaging drugs is efficiency of cellular DNA repair. While DNA repair is essential for a healthy organism’s survival, in tumor cells it can lead to drug resistance. Therefore, selective inhibition of DNA repair in cancer cells may greatly improve anti-tumor therapy.
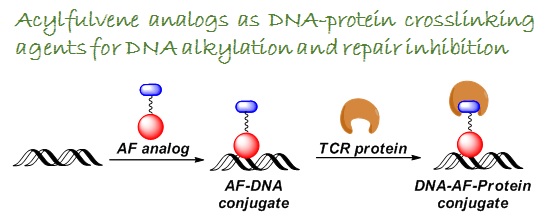 The strategy that is tested in our laboratory relies on developing bis-functional DNA alkylating agents that upon inducing DNA damage by alkyation, would also trigger inhibition of the repair pathway responsible for damage removal. The approach is based on utilizing a unique cancer-specific acylfulvene pharmacophores for developing new bioactive agents.
The strategy that is tested in our laboratory relies on developing bis-functional DNA alkylating agents that upon inducing DNA damage by alkyation, would also trigger inhibition of the repair pathway responsible for damage removal. The approach is based on utilizing a unique cancer-specific acylfulvene pharmacophores for developing new bioactive agents.
Synthesis of alkyl purines as mechanistic probes for testing the outcomes of DNA minor-groove alkylation
DNA minor-groove is an important receptor for enzymes and proteins involved in the processing and expression of genomic DNA. Sequences of high adenine/thymine content show several characteristic features, such as minor groove dimensions, hydrophobicity, electrostatics, hydration state, H-bond acceptors and donors, as well as duplex deformability, which distinguish them from random-sequence DNA tracts and contributes to the recognition by regulatory proteins. The outcome of disrupting these DNA-protein interactions by small molecules (natural products and their derivatives, active metabolites of environmental pollutants, or food components) can vary from induction of apoptosis (chemotherapy) or genotoxicity and carcinogenesis.
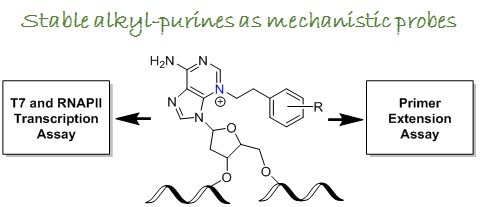 Our interest is to determine structural features that direct DNA damage to induce cell apoptosis. The major obstacle is that N3-alkylation of purines destabilizes glycosidic bond and results in a cleavage of DNA, thus limiting the use of direct conjugates in biochemical assays. The aim of this research project is to produce stable alkyl purines for analysis of their impact on DNA replication and transcription.
Our interest is to determine structural features that direct DNA damage to induce cell apoptosis. The major obstacle is that N3-alkylation of purines destabilizes glycosidic bond and results in a cleavage of DNA, thus limiting the use of direct conjugates in biochemical assays. The aim of this research project is to produce stable alkyl purines for analysis of their impact on DNA replication and transcription.