Research
The research in Karabencheva-Christova’s lab focuses on
Zn(II)-dependent matrix metalloproteinases (MMPs)
Hydrolysis of collagen – the most abundant protein in the human body and the main component of the extracellular matrix is a central biochemical process precisely regulated in normal physiology. However, abnormal collagen degradation underlines the development of numerous pathological conditions, including arthritis, fibrosis, inflammation and tumor growth, and metastasis. The primary type of enzymes performing collagen degradation is the Zn(II)-dependent matrix metalloproteinase (MMP) family, with one of its key members – the matrix metalloproteinase-1 (MMP-1). MMP-1 catalyzed collagen degradation involves significant conformational changes. By providing novel insights, through experimentally guided simulations, into the dynamics and catalytic mechanism of MMP-1 catalyzed collagen degradation, we aim to expand our knowledge of MMP-1 collagenolysis. NIH supports this research.
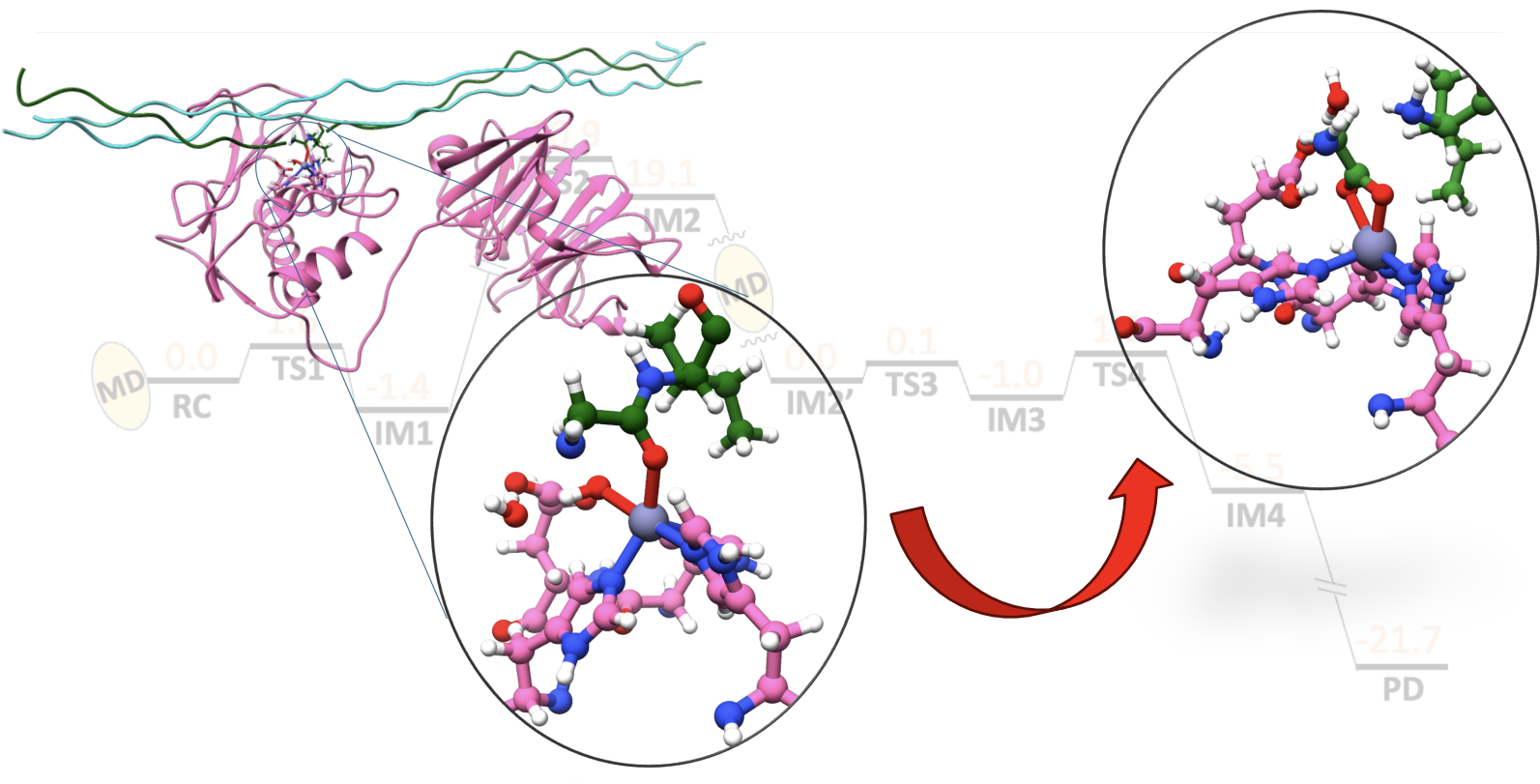
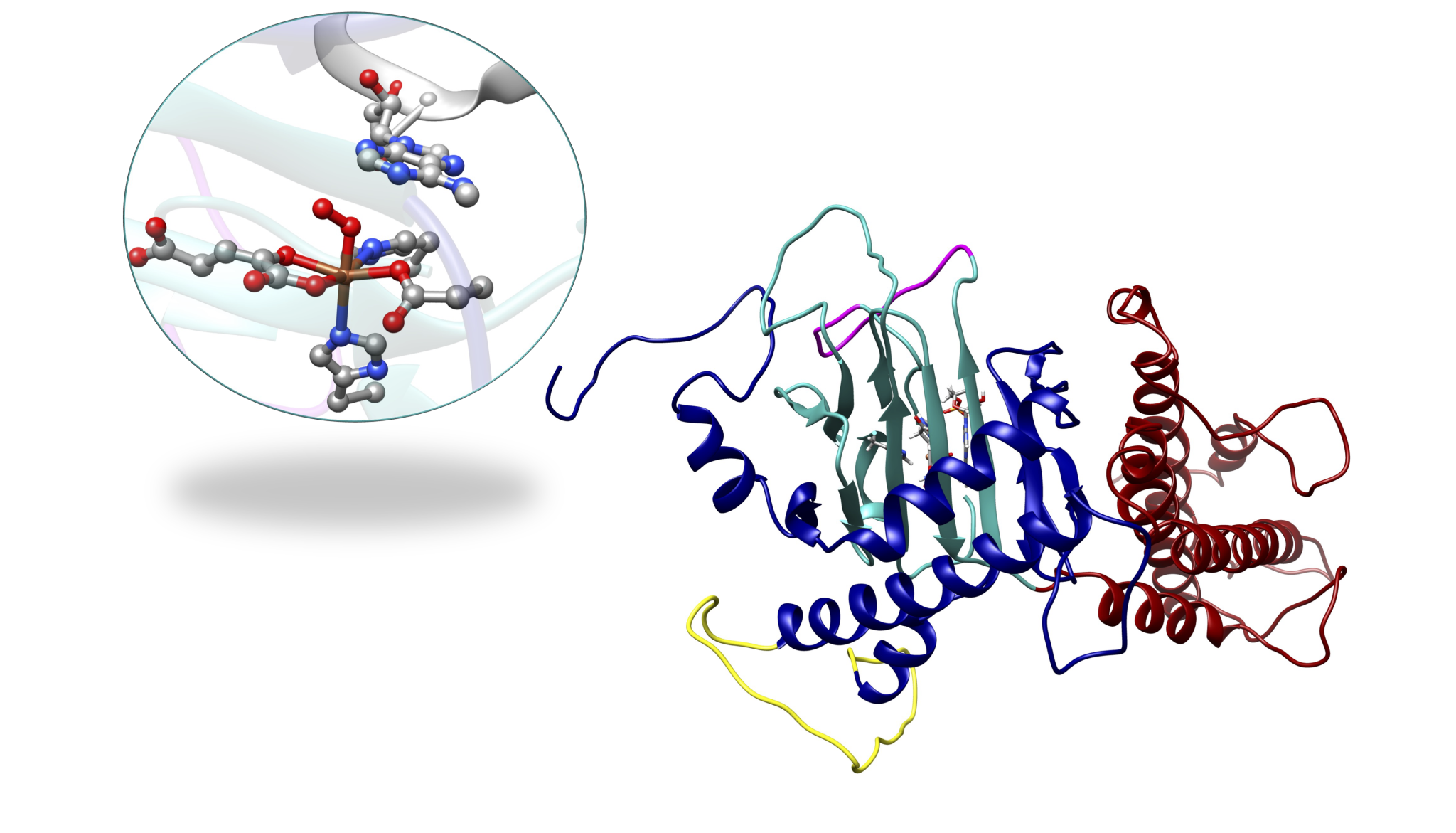
Non-heme Fe(II)- and 2-oxoglutarate (2OG) dependent oxygenases
Members of the non-heme Fe(II)- and 2-oxoglutarate (2OG)-dependent oxygenase family of enzymes catalyze diverse reactions with vital biological roles in cells such as hydroxylation; and demethylation; halogenation, desaturation, and others, making them attractive targets for therapeutics development. One of the essential roles of non-heme Fe(II)/2OG oxygenases is the demethylation of DNA and RNA bases. DNA and RNA are subject to exogenous and endogenous chemical modifications by a variety of alkylating agents. Reversible DNA/RNA methylation/demethylation modifications can regulate gene expression in epigenetics, therefore, revealing demethylation mechanisms is of key interest. A particular target of interest in our group is nucleic acid demethylases (NADMs). The NADMs perform hydroxylation of N-methylated DNA and RNA bases. Specifically, we study the bacterial AlkB enzyme, the human homologs- AlkBH2 and AlkBH5, and the human fat-mass and obesity-associated (FTO) protein.

